Welcome to your science lab internship!
You’ve been accepted to study cells and proteins at our science lab. Proteins fold in intricate ways to form structures that affect how they function. Let’s learn how scientists use highly advanced microscopes and techniques to study cell and protein structures that can keep you healthy—or contribute to diseases!
Click on any of the doors to start exploring.
Are Scientists Working in the Dark?
You can see a cell through a light microscope, but the tinier stuff—like proteins, DNA, or even viruses—are literally too small to see with light. So scientists had to invent new ways to see the molecular structures in our bodies.
Electrons vs. Light: When visible light waves bounce off objects, they are illuminated at a wavelength that human eyes see. Electrons have a smaller wavelength than light, so while humans can’t detect electrons’ brightness, special electron microscopes can! These microscopes use electrons to illuminate specimens that are too small to interact with light, but do interact with electrons.
Click on glowing objects to learn about them and unlock other items.
Frozen Proteins
Before using a cryo-electron microscope (cryo-EM), scientists flash-freeze a specimen, like a protein, by plunging it into liquid ethane to freeze and preserve it. This puts the specimen into a clear, glasslike state (unlike the opaque, white ice you sometimes see in your freezer).
Cryo- means extreme cold—that’s where this technique gets its name!
LET'S ASK A SCIENTIST!Dr. Melody Campbell is a biophysicist researching how cells communicate and interact with their surroundings. The goal of her lab’s research is to influence future drugs for autoimmune diseases and cancer.
Dr. Campbell, how do you prepare
samples for cryo-EM?
We put our samples on a very small circular grid made of copper or gold. The grid is smaller than the size of a pencil eraser, and we use just a tiny drop of sample: 3 microliters, which is less than a raindrop.
Like a raindrop, the sample is normally clear and seems motionless to the eye, but there are millions of tiny proteins in the drop that are constantly moving around and interacting—a whole invisible world about to be opened up by cryo-EM!
We preserve these samples by freezing them at an ultracold temperature (about -320˚F), many times colder than your freezer at home, and we keep the samples at this temperature
even when they are inside the
microscope.
 It’s incredible that even though these proteins are so, so tiny, technology has allowed us to see them in extremely clear detail, which helps us learn how they function.
It’s incredible that even though these proteins are so, so tiny, technology has allowed us to see them in extremely clear detail, which helps us learn how they function.
Electron Blaster
Once scientists have the frozen specimen ready, they put it in an electron microscope to blast it with electrons. A specialized camera detects how the electrons bounce off the atoms in the specimen, which tells scientists where the atoms are located.
LET'S ASK A SCIENTIST! Dr. Campbell, why do you use cryo-EM and not a light microscope?
Dr. Campbell, why do you use cryo-EM and not a light microscope?
The setup of an electron microscope is similar to a more-familiar light microscope (like the ones you may have used at school). Just like in a light microscope, the electron microscope has a source of illumination, a stage where the sample sits, and lenses that are used to control the illumination and magnify the sample. However, in an electron microscope, the illumination comes from electrons instead of light, and the lenses are electromagnetic and made of copper coils instead of glass.
A bigger microscope for smaller specimens: Electron microscopes are much larger than light microscopes. Some electron microscopes are over 10 feet tall and weigh as much as a hippopotamus!
The electron microscope allows us to magnify samples far over a million times, whereas light microscopes are limited at about 1,500x. This lets us view samples that are smaller than the wavelength of light, such as viruses, DNA, RNA, and individual proteins. For the type of electron microscopy I do, most bacteria are actually too large to look at!
Image Mapping
Cryo-electron microscopes use a specialized camera to record images of samples. A computer processes these images, layering hundreds of thousands of images of individual molecules or atoms to create a map of the protein’s 3D shape and structure.
LET'S ASK A SCIENTIST!SEE 3D IMAGE MAPS!Why are computers essential
for cryo-EM?
 After we collect millions of two-dimensional protein images, we have to process and analyze this data. To do this we use a “cluster” of computers, which can be thought of as many—sometimes hundreds—of computers all connected to each other that work together to do the same task.
After we collect millions of two-dimensional protein images, we have to process and analyze this data. To do this we use a “cluster” of computers, which can be thought of as many—sometimes hundreds—of computers all connected to each other that work together to do the same task.
If we tried to run some of our software programs on a standard desktop computer like the ones you probably have at school, it could take years to complete.
We use the software to interpret and transform the two-dimensional images into three-dimensional maps of the specimen’s structure.
SEE 3D IMAGE MAPS!Dr. Christopher Barnes uses cryo-EM to research how viral proteins infect human cells. You’ll also meet him again in another room!
Dr. Barnes, why do you use cryo-EM to look at proteins?
Rarely do proteins act alone when carrying out biological processes. A good analogy would be like looking at a clock (i.e., a cell). From the outside, we see the hands of the clock moving, telling us the time. However, internally this clock is very complex, with many gears (i.e., proteins) that must work together.
Even if only one gear stops working, that can be problematic for an accurate telling of time (or the function of the cell). So much like a clock engineer, as a structural biologist, I want to understand how all these proteins work together.
The beauty of cryo-EM is that we can use it to see, at the molecular level, the complexity of these biological systems. Rather than focusing on one protein alone, we can create an image map of how multiple proteins fit together to carry out a task.
Dr. Melody Campbell
Assistant Professor, The Fred Hutchinson
Cancer Research Center
Type of Scientist: Biophysicist, using physics, chemistry, and math to research how cells communicate
Did you know what you wanted to do or be when you left high school?
No! I started university taking music courses as well as premed courses. It was not a straight path from high school to graduate school, but I was grateful to go to a large public university, which required me to take a variety of classes to explore what I wanted most.
Which accomplishment are you proudest of?
There was a group of us working with a new type of electron microscope camera. After several years of trying, writing software, and collecting lots of data, we determined the first structure of a protein in such high detail that you could actually “see” water molecules near the surface. Before that, it was not known if cryo-EM could ever see in enough detail to do that!
Invisible Rays
Have you ever gotten X-rays of your teeth at the dentist? Your eyes
wouldn’t have been able to see the X-rays firing at your mouth, but a
machine detected how the X-rays bounced off your teeth, revealing any
holes in a tooth’s structure (uh-oh, a cavity).
Scientists also use X-rays to reveal the structure of things much smaller than
teeth. In fact, X-ray crystallography was the very first technique used to reveal
the structure of a protein—and it’s still used today.
Click on glowing objects to learn about them and unlock other items.
Different Tools for Different
Investigations
X-ray crystallography only works on specimens that can be crystallized. Unfortunately, not all proteins can be crystallized.
A lab that studies ion channels (more details below) uses a wide range of cutting-edge approaches, since each has benefits and limitations. The scientists use cryo-EM, X-ray crystallography, electrical recording tools, and other techniques as they investigate the body’s processes to help make better medicines.
What are ion channels, anyway? Ions are electrically charged particles. Electricity helps your heart beat and your brain cells to function. Ion channels are proteins that create tiny, momentary holes in cell membranes. Charged ions move through the holes, generating an electric current that allows cells to send messages to each other, including, “Move now!” and “This hurts!”
LET'S ASK A SCIENTIST!Dr. Sudha Chakrapani is a structural biologist, researching the electrical communication between the brain and body.

Dr. Chakrapani, why do you need to use multiple imaging techniques?
The ion channels we study are relatively small proteins that are highly dynamic. These proteins reconfigure to change shape depending on what function they’re performing. They have been difficult to purify in large quantities and are incompatible with X-ray crystallography.
Unlike X-ray crystallography, cryo-EM methods don’t require the protein to be crystallized. As a result, cryo-EM has enabled us to capture the ion channel in various functional states.
Crystallized Proteins
 Is this a stained-glass window? No, these are crystals of proteins, which scientists grow as a first step to solving the mystery of their structures. First, scientists purify the protein, using a series of procedures to separate their target protein from the cells or tissues it’s in. Then, using chemistry, scientists prompt the protein sample to grow into a highly organized form called a crystal, which can take months or even years.
Is this a stained-glass window? No, these are crystals of proteins, which scientists grow as a first step to solving the mystery of their structures. First, scientists purify the protein, using a series of procedures to separate their target protein from the cells or tissues it’s in. Then, using chemistry, scientists prompt the protein sample to grow into a highly organized form called a crystal, which can take months or even years.
A crystal of cow trypsin protein: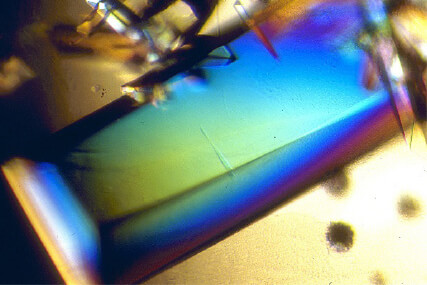
Crystals of pig alpha amylase protein: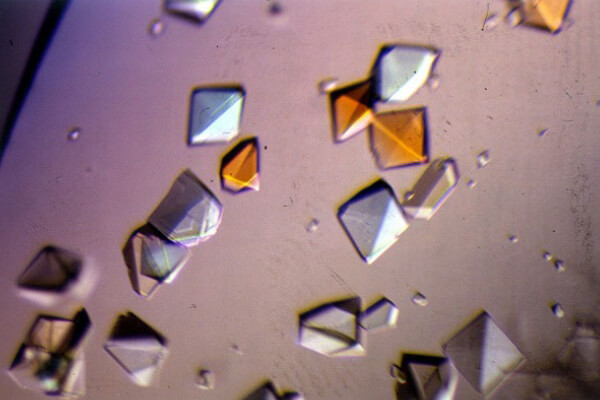
Growing crystals takes months, but not all crystals will produce usable results. Scientists use an automated crystal screening system to rapidly screen samples to identify the best candidates for further study.
Cool Fact: Some proteins are crystallized on the International Space Station because the microgravity there allows larger and more well-ordered crystals to grow!
X-Ray Diffraction
Once scientists have a viable crystal of a specimen, they fire X-rays through it. The X-rays hit the individual atoms in the protein crystal and bend in different directions (called diffraction), forming a dot pattern on a detector.
Then, scientists use a formula and computer software to infer what 3D structure would cause that dot pattern.
Sudha Chakrapani, Ph.D.
Professor, Case Western Reserve University
Type of Scientist: Structural biologist, researching how the brain communicates with the body
How does your work help people?
What we study could directly affect human health, including diseases that currently have no cure. Solving protein structures to the atomic level gives you a better understanding of how they function in good health and what goes wrong when a disease-causing mutation is present.
When you know the protein’s structure, you may find an ideal location for targeting a medicine. This serves as a blueprint for designing a more effective treatment, with minimal side effects.
Our lab recently solved the first cryo-EM structures for a type of serotonin receptor. We went on to show how blocking this serotonin channel [with a medicine] could help treat nausea and vomiting in cancer patients.
Right: A 3D cryo-EM reconstruction of an antinausea drug (shown in orange) bound together with a receptor (in gray) for serotonin, a neurotransmitter that sends messages in the brain.
Image courtesy of Sudha Chakrapani, Case Western Reserve University School of Medicine.
Unlocking Protein Mysteries
There are many, many different types of proteins, and often their structures remain a mystery—which makes it harder for scientists to study diseases and treatments. Adding to the puzzle, about 25 percent of total proteins don’t crystallize in a way that allows them to be viewed by typical X-ray crystallography techniques. That’s why the X-ray free electron laser, a relatively new tool, is attracting attention!
What’s a laser? A laser is a tool that concentrates light over and over again until it lines up in a really powerful, concentrated light beam. That’s why laser beams are so thin, unlike the light that comes out of a lightbulb in every direction.
Click on glowing objects to learn about them and unlock other items.
While Dr. Christopher Barnes mainly uses cryo-EM for his research on how viral proteins infect human cells, he has also had the opportunity to use the uncommon X-ray free electron laser.
Dr. Barnes, what’s it like using an X-ray free electron laser?
It’s so cool! This is a multibillion-dollar instrument that I get to control. A few years ago, when I was using the X-ray free electron laser at Stanford, there were only two of these instruments operational in the world. So, when I was sitting at the control panel, at that moment in time, I was one of only two scientists in the world doing this type of work.
The joy that comes when the data collected results in a new structure is one of the most satisfying feelings one can have as a structural biologist.
A Laser That Travels Over a Mile
An X-ray free electron laser emits a laser beam that is way, way, way more intense than a typical laser. That makes it much brighter, allowing scientists to capture images of matter down at the atomic level! (Remember, everything physical in the universe is made up of molecules, which are made up of atoms.)
This tool is generally over a mile long and requires a massive underground tunnel. Its workings are complicated, but in short: While emitting pulses (short bursts) of electrons, an X-ray free electron laser uses a special magnetic field that forces the electrons onto their path.
Because the pulses of electrons are so bright and short, the tool can take pictures of molecules before radiation destroys them. Unlike most techniques, this tool can even capture chemical processes while they are taking place!
Christopher Barnes, Ph.D.
Postdoctoral Fellow, California Institute of Technology
Type of Scientist: Structural biologist, researching how viral proteins infect human cells
Did you know what you wanted to do or be when you left high school?
Entering college, I aspired to be a medical doctor (M.D.), having no understanding of what it meant to obtain a Ph.D. degree. As a double major (chemistry and psychology) and football player, my GPA suffered. To strengthen my weak academic profile, I reached out to the professors in the chemistry department to find a lab I could join as an undergraduate research technician. It was here that I first began to understand the possibility of this becoming a career.
How did reaching out to the professor who became your mentor ultimately help you decide on your career?
My mentor once told me, “Why be an M.D. who for the most part repeats procedures day to day, when you can be a Ph.D. and discover the cures and treatments that those M.D.s prescribe?” At that moment, I decided to pursue my Ph.D. I owe my career trajectory to my mentor because it was his behind-the-scenes support and belief in me that allowed me to prove to myself (and later, others) that I was capable of conducting research at a high level
What would you like to contribute to your field? What are you proud of?
I hope to advance our understanding of interactions between viruses and hosts. I hope this information can someday lead to an effective vaccine against HIV-1 (the virus that causes AIDS).
As a scientist, I have always wanted to help the world with my research, including with my work on the virus that causes COVID-19. The opportunity to provide some of the first structures of human antibodies interacting with that virus’s spike protein was a special moment.
Seeing Through Objects
We can’t see through walls, but a confocal laser scanning microscope (CLSM) can focus its laser at different depths in a specimen—in other words, it can see inside without the specimen having to be cut open! For specimens that are difficult to slice to create cross-sectional views, a CLSM is perfect! It combines concepts from the light microscope and the laser to give us groundbreaking images.
Click on glowing objects to learn about them and unlock other items.
Light Microscope
If you’ve used a microscope in school, it’s probably a light microscope, which uses regular light to illuminate a specimen (unlike newer imaging techniques).
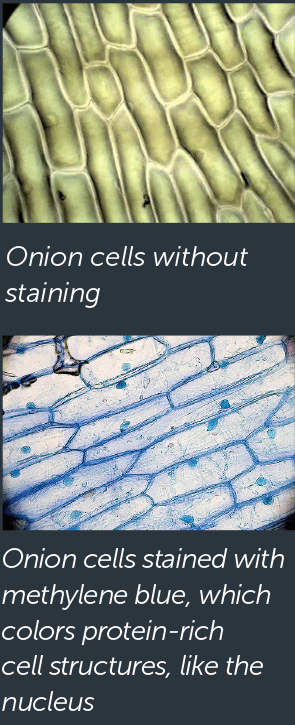
 How It Works: Light microscopes use curved glass lenses in order to bend light, which makes an object look bigger than it is. The light microscopes commonly used in schools magnify up to about 400 times actual size. Although today’s advanced microscopes can magnify much more, the first light microscope was a revolutionary scientific tool when it was invented in 1595.
How It Works: Light microscopes use curved glass lenses in order to bend light, which makes an object look bigger than it is. The light microscopes commonly used in schools magnify up to about 400 times actual size. Although today’s advanced microscopes can magnify much more, the first light microscope was a revolutionary scientific tool when it was invented in 1595.
Benefits and Limitations: Although a light microscope can’t magnify as much as other types of microscopes (so it can’t see individual proteins, for example), it has the advantage of being able to magnify living cells—so it’s possible to find out not just a cell’s shape, but some information about how it acts.
What is your strategy when you have a tough problem to solve
or a challenge to overcome?
 Dr. Shraddha Nayak: First breathe and then look online. If you don’t find solutions, see if you have approached the problem from different angles. If still no solution, take a break. Go for a walk.
Dr. Shraddha Nayak: First breathe and then look online. If you don’t find solutions, see if you have approached the problem from different angles. If still no solution, take a break. Go for a walk.
 Dr. Christopher Barnes: Communicating and asking your peers or mentors for help is the easiest—but oftentimes most underutilized—strategy for confronting challenges in the lab or even life.
Dr. Christopher Barnes: Communicating and asking your peers or mentors for help is the easiest—but oftentimes most underutilized—strategy for confronting challenges in the lab or even life.
 Dr. Sudha Chakrapani: I break a challenge down into small steps or goals. My approach to science has evolved and benefitted from my exposure to a diverse scientific network—scientists with wide-ranging backgrounds who bring unique perspectives.
Dr. Sudha Chakrapani: I break a challenge down into small steps or goals. My approach to science has evolved and benefitted from my exposure to a diverse scientific network—scientists with wide-ranging backgrounds who bring unique perspectives.
 Dr. Melody Campbell: First I read. Maybe there was some background information I missed or some experiments that have already been done that could help me solve it. Next, I ask for help! There is far too much science in the world for any one person to know everything.
Dr. Melody Campbell: First I read. Maybe there was some background information I missed or some experiments that have already been done that could help me solve it. Next, I ask for help! There is far too much science in the world for any one person to know everything.
Focused Light
Confocal means the light is focused in one place. The microscope forces the light through a pinhole (a tiny hole) to block out-of-focus light when capturing the image of the specimen, which increases the resolution without blurriness. (Since this microscope uses light and not electrons or X-rays, it’s ideal for visualizing cells or tissues, but not molecules.)
Instead of using a lightbulb (like a light microscope you’d use in school), this microscope uses light from a laser that’s focused with a lens. Then, mirrors move to reflect the laser and guide it across the specimen (scanning). If fluorescence (molecules that glow) has been added to the specimen, the microscope’s detectors pick up that light to help capture the image. The microscope takes photos of each depth, then software stitches the photos together into an image of 3D structures.
Science in Motion
Scientific research relies on many different skills to interpret, visualize, and demonstrate data. Molecular animators create animations of data from many types of microscopes and imaging techniques to help scientists (and others) better understand biological processes.
LET'S ASK A SCIENTIST!SEE SCIENCE ANIMATIONSDr. Shraddha Nayak is a molecular animator, using graphic design software, coding, and structural and biochemical data to create visualizations.
Dr. Nayak, why is molecular animation helpful?
The 3D animation software I use has mind-blowing capabilities! It lets me create a setting inside or outside a cell, simulate a process that is taking place, and show movements and changes over time. The tools I work with allow scientists to see their hypotheses and communicate their findings.
SEE SCIENCE ANIMATIONSShraddha Nayak, Ph.D.
Postdoctoral Fellow, University of Utah
Type of Scientist: Molecular animator, using 3D animation and graphic design software, coding, and structural and biochemical data to create visualizations
Did you know what you wanted to do or be when you left high school?
No, I had no clue I wanted to do scientific visualization [animating molecules] until before defending my Ph.D. thesis in pharmacology, which is the study of medications and their actions. Growing up in India, we were generally encouraged to study the sciences. Luckily, I enjoyed biology, especially the nervous system, when I was in high school but didn’t really explore paths I could take. And now I collaborate with neuroscientists to make animations. Looking back, I do wish I had explored earlier, and pursued more art and computer science as well.
What are the coolest parts of your day as a scientist?
Every part is cool, but I really enjoy the variety of projects we work on. There’s no chance to be bored, since there’s always some challenge waiting around the corner.
One of the most fascinating things is seeing the excitement on the faces of your collaborators when a visualization helped them “see” something important or it made a difference to their research or communication. I’m just proud to be able to do what I really enjoy and have multiple skills as a scientist, designer, artist, and programmer.
Job Well Done!
Wow, there are so many skills you can use as a scientist! Before you go, think about how you could use important skills like organization, writing, collaboration, and design in a science field. Then read the scientists’ advice for future careers.




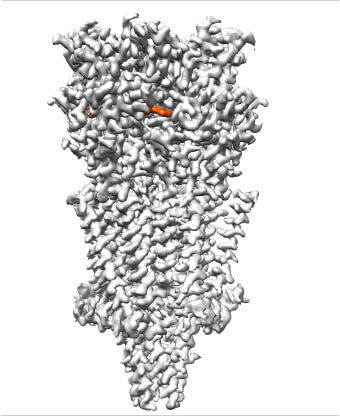 Right: A 3D cryo-EM reconstruction of an antinausea drug (shown in orange) bound together with a receptor (in gray) for serotonin, a neurotransmitter that sends messages in the brain.
Right: A 3D cryo-EM reconstruction of an antinausea drug (shown in orange) bound together with a receptor (in gray) for serotonin, a neurotransmitter that sends messages in the brain.