Switch to List View
Image and Video Gallery
This is a searchable collection of scientific photos, illustrations, and videos. The images and videos in this gallery are licensed under Creative Commons Attribution Non-Commercial ShareAlike 3.0. This license lets you remix, tweak, and build upon this work non-commercially, as long as you credit and license your new creations under identical terms.
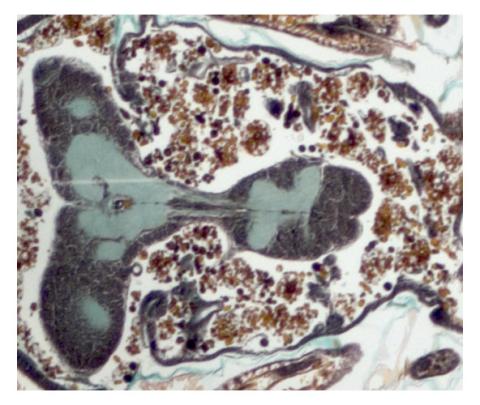
2758: Cross section of a Drosophila melanogaster pupa
2758: Cross section of a Drosophila melanogaster pupa
This photograph shows a magnified view of a Drosophila melanogaster pupa in cross section. Compare this normal pupa to one that lacks an important receptor, shown in image 2759.
Christina McPhee and Eric Baehrecke, University of Massachusetts Medical School
View Media
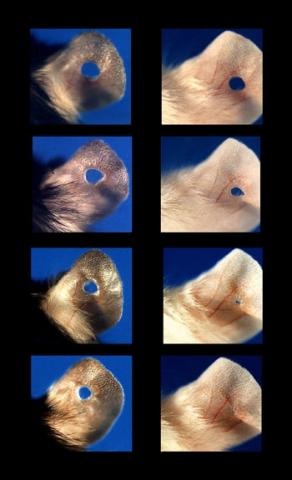
3426: Regeneration of Mouse Ears
3426: Regeneration of Mouse Ears
Normal mice, like the B6 breed pictured on the left, develop scars when their ears are pierced. The Murphy Roths Large (MRL) mice pictured on the right can grow back lost ear tissue thanks to an inactive version of the p21 gene. When researchers knocked out that same gene in other mouse breeds, their ears also healed completely without scarring. Journal Article: Clark, L.D., Clark, R.K. and Heber-Katz, E. 1998. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol 88: 35-45.
Ellen Heber-Katz, The Wistar Institute
View Media
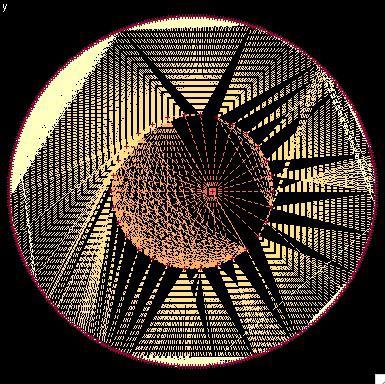
2322: Modeling disease spread
2322: Modeling disease spread
What looks like a Native American dream catcher is really a network of social interactions within a community. The red dots along the inner and outer circles represent people, while the different colored lines represent direct contact between them. All connections originate from four individuals near the center of the graph. Modeling social networks can help researchers understand how diseases spread.
Stephen Eubank, University of Virginia Biocomplexity Institute (formerly Virginia Bioinformatics Institute)
View Media
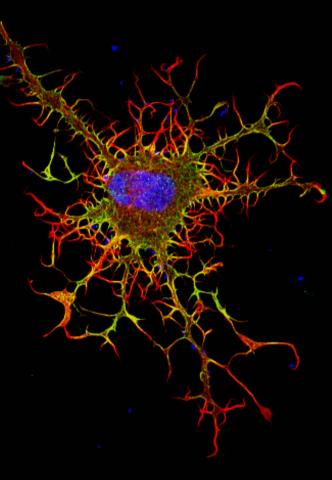
3613: Abnormal, spiky fibroblast
3613: Abnormal, spiky fibroblast
This is a fibroblast, a connective tissue cell that plays an important role in wound healing. Normal fibroblasts have smooth edges. In contrast, this spiky cell is missing a protein that is necessary for proper construction of the cell's skeleton. Its jagged shape makes it impossible for the cell to move normally. In addition to compromising wound healing, abnormal cell movement can lead to birth defects, faulty immune function, and other health problems.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
Praveen Suraneni, Stowers Institute for Medical Research, Kansas City, Mo.
View Media
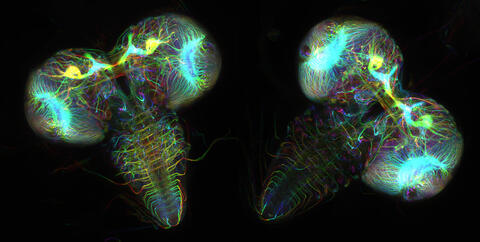
6808: Fruit fly larvae brains showing tubulin
6808: Fruit fly larvae brains showing tubulin
Two fruit fly (Drosophila melanogaster) larvae brains with neurons expressing fluorescently tagged tubulin protein. Tubulin makes up strong, hollow fibers called microtubules that play important roles in neuron growth and migration during brain development. This image was captured using confocal microscopy, and the color indicates the position of the neurons within the brain.
Vladimir I. Gelfand, Feinberg School of Medicine, Northwestern University.
View Media
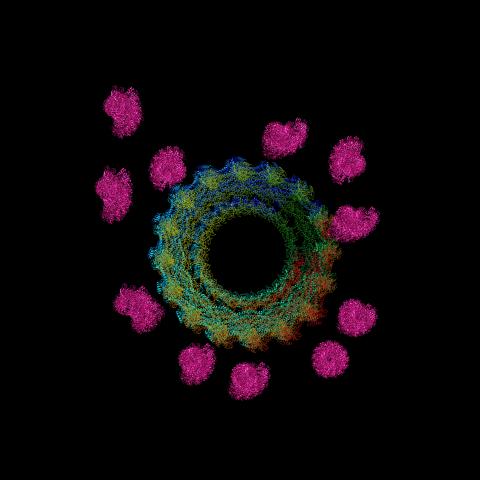
6571: Actin filaments bundled around the dynamin helical polymer
6571: Actin filaments bundled around the dynamin helical polymer
Multiple actin filaments (magenta) are organized around a dynamin helical polymer (rainbow colored) in this model derived from cryo-electron tomography. By bundling actin, dynamin increases the strength of a cell’s skeleton and plays a role in cell-cell fusion, a process involved in conception, development, and regeneration.
Elizabeth Chen, University of Texas Southwestern Medical Center.
View Media
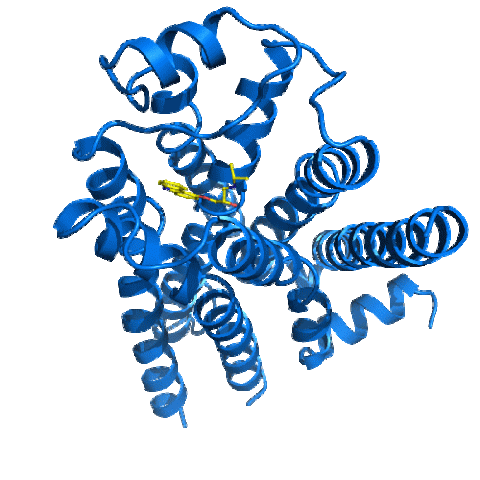
2337: Beta2-adrenergic receptor protein
2337: Beta2-adrenergic receptor protein
Crystal structure of the beta2-adrenergic receptor protein. This is the first known structure of a human G protein-coupled receptor, a large family of proteins that control critical bodily functions and the action of about half of today's pharmaceuticals. Featured as one of the November 2007 Protein Structure Initiative Structures of the Month.
The Stevens Laboratory, The Scripps Research Institute
View Media
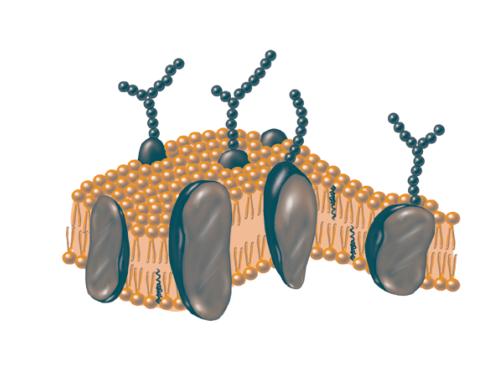
2523: Plasma membrane
2523: Plasma membrane
The plasma membrane is a cell's protective barrier. See image 2524 for a labeled version of this illustration. Featured in The Chemistry of Health.
Crabtree + Company
View Media
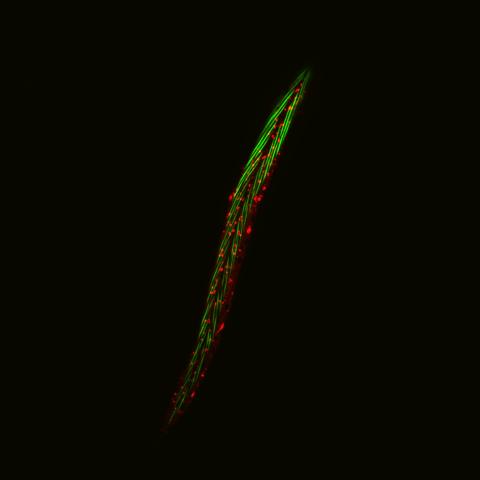
6581: Fluorescent C. elegans showing muscle and ribosomal protein
6581: Fluorescent C. elegans showing muscle and ribosomal protein
C. elegans, a tiny roundworm, with a ribosomal protein glowing red and muscle fibers glowing green. Researchers used these worms to study a molecular pathway that affects aging. The ribosomal protein is involved in protein translation and may play a role in dietary restriction-induced longevity. Image created using confocal microscopy.
View group of roundworms here 6582.
View closeup of roundworms here 6583.
View group of roundworms here 6582.
View closeup of roundworms here 6583.
Jarod Rollins, Mount Desert Island Biological Laboratory.
View Media
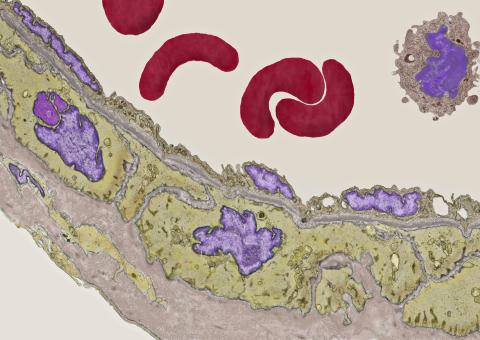
3738: Transmission electron microscopy of coronary artery wall with elastin-rich ECM pseudocolored in light brown
3738: Transmission electron microscopy of coronary artery wall with elastin-rich ECM pseudocolored in light brown
Elastin is a fibrous protein in the extracellular matrix (ECM). It is abundant in artery walls like the one shown here. As its name indicates, elastin confers elasticity. Elastin fibers are at least five times stretchier than rubber bands of the same size. Tissues that expand, such as blood vessels and lungs, need to be both strong and elastic, so they contain both collagen (another ECM protein) and elastin. In this photo, the elastin-rich ECM is colored grayish brown and is most visible at the bottom of the photo. The curved red structures near the top of the image are red blood cells.
Tom Deerinck, National Center for Microscopy and Imaging Research (NCMIR)
View Media
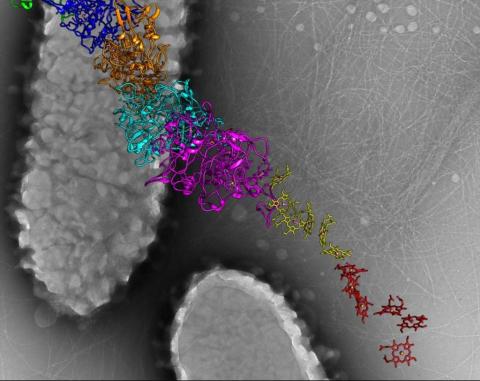
6580: Bacterial nanowire model
6580: Bacterial nanowire model
A model of a Geobacter sulfurreducens nanowire created from cryo-electron microscopy images. The bacterium conducts electricity through these nanowires, which are made up of protein and iron-containing molecules.
Edward Egelman, University of Virginia.
View Media
6553: Floral pattern in a mixture of two bacterial species, Acinetobacter baylyi and Escherichia coli, grown on a semi-solid agar for 48 hours (photo 1)
6553: Floral pattern in a mixture of two bacterial species, Acinetobacter baylyi and Escherichia coli, grown on a semi-solid agar for 48 hours (photo 1)
Floral pattern emerging as two bacterial species, motile Acinetobacter baylyi (red) and non-motile Escherichia coli (green), are grown together for 48 hours on 1% agar surface from a small inoculum in the center of a Petri dish.
See 6557 for a photo of this process at 24 hours on 0.75% agar surface.
See 6555 for another photo of this process at 48 hours on 1% agar surface.
See 6556 for a photo of this process at 72 hours on 0.5% agar surface.
See 6550 for a video of this process.
See 6557 for a photo of this process at 24 hours on 0.75% agar surface.
See 6555 for another photo of this process at 48 hours on 1% agar surface.
See 6556 for a photo of this process at 72 hours on 0.5% agar surface.
See 6550 for a video of this process.
L. Xiong et al, eLife 2020;9: e48885
View Media
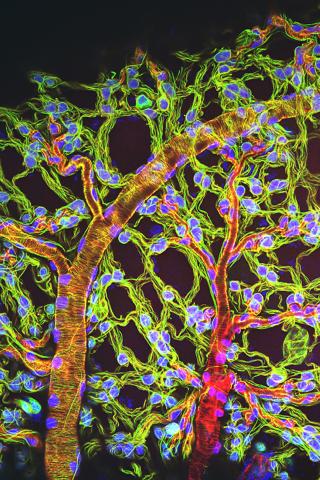
3616: Weblike sheath covering developing egg chambers in a giant grasshopper
3616: Weblike sheath covering developing egg chambers in a giant grasshopper
The lubber grasshopper, found throughout the southern United States, is frequently used in biology classes to teach students about the respiratory system of insects. Unlike mammals, which have red blood cells that carry oxygen throughout the body, insects have breathing tubes that carry air through their exoskeleton directly to where it's needed. This image shows the breathing tubes embedded in the weblike sheath cells that cover developing egg chambers.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
Kevin Edwards, Johny Shajahan, and Doug Whitman, Illinois State University.
View Media
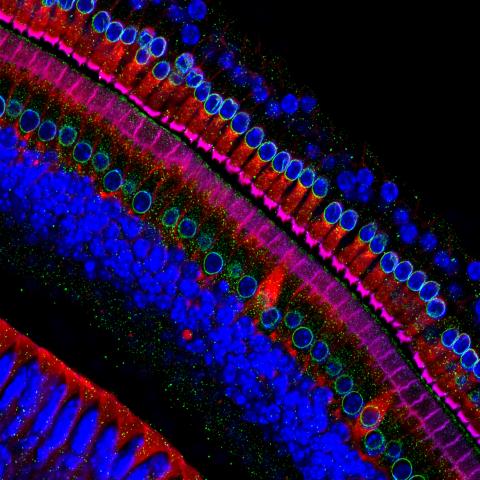
3618: Hair cells: the sound-sensing cells in the ear
3618: Hair cells: the sound-sensing cells in the ear
These cells get their name from the hairlike structures that extend from them into the fluid-filled tube of the inner ear. When sound reaches the ear, the hairs bend and the cells convert this movement into signals that are relayed to the brain. When we pump up the music in our cars or join tens of thousands of cheering fans at a football stadium, the noise can make the hairs bend so far that they actually break, resulting in long-term hearing loss.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
Henning Horn, Brian Burke, and Colin Stewart, Institute of Medical Biology, Agency for Science, Technology, and Research, Singapore
View Media
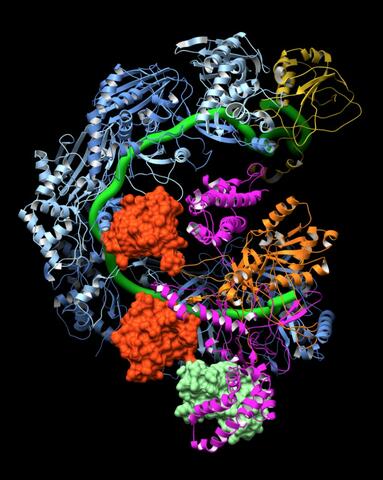
6352: CRISPR surveillance complex
6352: CRISPR surveillance complex
This image shows how the CRISPR surveillance complex is disabled by two copies of anti-CRISPR protein AcrF1 (red) and one AcrF2 (light green). These anti-CRISPRs block access to the CRISPR RNA (green tube) preventing the surveillance complex from scanning and targeting invading viral DNA for destruction.
NRAMM National Resource for Automated Molecular Microscopy http://nramm.nysbc.org/nramm-images/ Source: Bridget Carragher
View Media
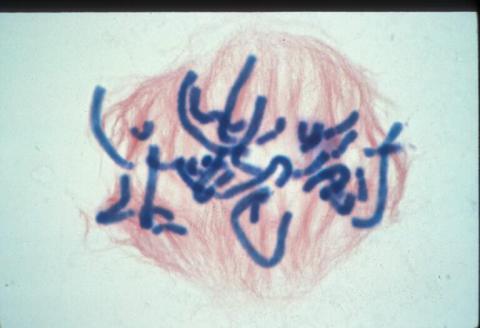
1016: Lily mitosis 06
1016: Lily mitosis 06
A light microscope image of a cell from the endosperm of an African globe lily (Scadoxus katherinae). This is one frame of a time-lapse sequence that shows cell division in action. The lily is considered a good organism for studying cell division because its chromosomes are much thicker and easier to see than human ones. Staining shows microtubules in red and chromosomes in blue. Here, condensed chromosomes are clearly visible and are starting to line up.
Related to images 1010, 1011, 1012, 1013, 1014, 1015, 1017, 1018, 1019, and 1021.
Related to images 1010, 1011, 1012, 1013, 1014, 1015, 1017, 1018, 1019, and 1021.
Andrew S. Bajer, University of Oregon, Eugene
View Media
1083: Natcher Building 03
1083: Natcher Building 03
NIGMS staff are located in the Natcher Building on the NIH campus.
Alisa Machalek, National Institute of General Medical Sciences
View Media
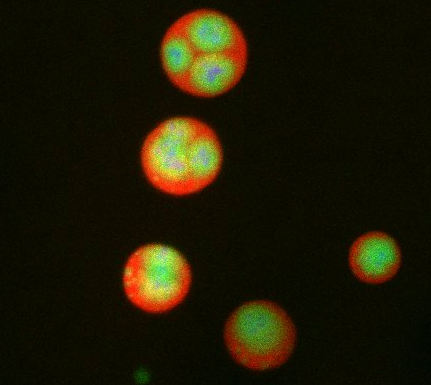
3792: Nucleolus subcompartments spontaneously self-assemble 3
3792: Nucleolus subcompartments spontaneously self-assemble 3
What looks a little like distant planets with some mysterious surface features are actually assemblies of proteins normally found in the cell's nucleolus, a small but very important protein complex located in the cell's nucleus. It forms on the chromosomes at the location where the genes for the RNAs are that make up the structure of the ribosome, the indispensable cellular machine that makes proteins from messenger RNAs.
However, how the nucleolus grows and maintains its structure has puzzled scientists for some time. It turns out that even though it looks like a simple liquid blob, it's rather well-organized, consisting of three distinct layers: the fibrillar center, where the RNA polymerase is active; the dense fibrillar component, which is enriched in the protein fibrillarin; and the granular component, which contains a protein called nucleophosmin. Researchers have now discovered that this multilayer structure of the nucleolus arises from differences in how the proteins in each compartment mix with water and with each other. These differences let the proteins readily separate from each other into the three nucleolus compartments.
This photo of nucleolus proteins in the eggs of a commonly used lab animal, the frog Xenopus laevis, shows each of the nucleolus compartments (the granular component is shown in red, the fibrillarin in yellow-green, and the fibrillar center in blue). The researchers have found that these compartments spontaneously fuse with each other on encounter without mixing with the other compartments.
For more details on this research, see this press release from Princeton. Related to video 3789, video 3791 and image 3793.
However, how the nucleolus grows and maintains its structure has puzzled scientists for some time. It turns out that even though it looks like a simple liquid blob, it's rather well-organized, consisting of three distinct layers: the fibrillar center, where the RNA polymerase is active; the dense fibrillar component, which is enriched in the protein fibrillarin; and the granular component, which contains a protein called nucleophosmin. Researchers have now discovered that this multilayer structure of the nucleolus arises from differences in how the proteins in each compartment mix with water and with each other. These differences let the proteins readily separate from each other into the three nucleolus compartments.
This photo of nucleolus proteins in the eggs of a commonly used lab animal, the frog Xenopus laevis, shows each of the nucleolus compartments (the granular component is shown in red, the fibrillarin in yellow-green, and the fibrillar center in blue). The researchers have found that these compartments spontaneously fuse with each other on encounter without mixing with the other compartments.
For more details on this research, see this press release from Princeton. Related to video 3789, video 3791 and image 3793.
Nilesh Vaidya, Princeton University
View Media
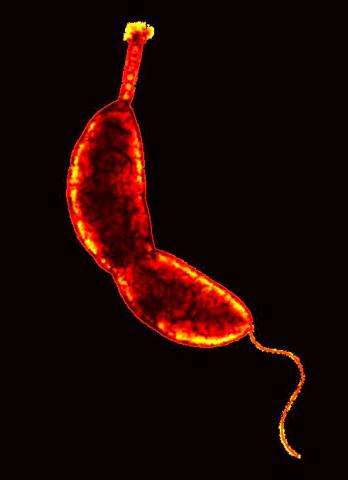
3262: Caulobacter
3262: Caulobacter
A study using Caulobacter crescentus showed that some bacteria use just-in-time processing, much like that used in industrial delivery, to make the glue that allows them to attach to surfaces, an important step in the infection process for many disease-causing bacteria. In the image shown, this freshwater bacterium has a holdfast at the top and a propelling flagellum at the end. From an Indiana University news release.
Yves Brun, Indiana University
View Media
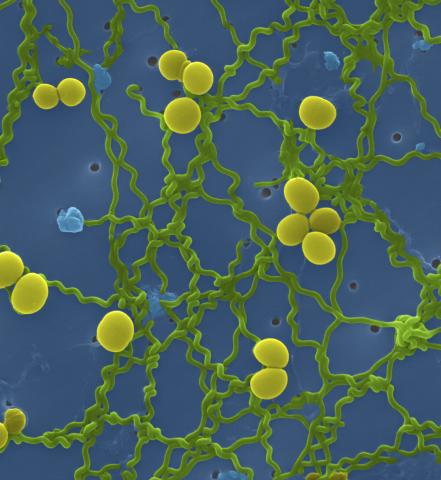
1166: Leptospira bacteria
1166: Leptospira bacteria
Leptospira, shown here in green, is a type (genus) of elongated, spiral-shaped bacteria. Infection can cause Weil's disease, a kind of jaundice, in humans.
Tina Weatherby Carvalho, University of Hawaii at Manoa
View Media

1060: Protein crystals
1060: Protein crystals
Structural biologists create crystals of proteins, shown here, as a first step in a process called X-ray crystallography, which can reveal detailed, three-dimensional protein structures.
Alex McPherson, University of California, Irvine
View Media
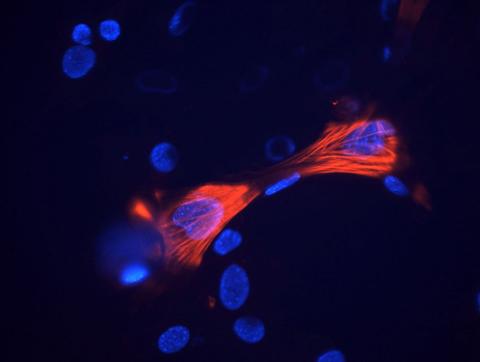
3289: Smooth muscle from mouse stem cells
3289: Smooth muscle from mouse stem cells
These smooth muscle cells were derived from mouse neural crest stem cells. Red indicates smooth muscle proteins, blue indicates nuclei. Image and caption information courtesy of the California Institute for Regenerative Medicine.
Deepak Srivastava, Gladstone Institutes, via CIRM
View Media
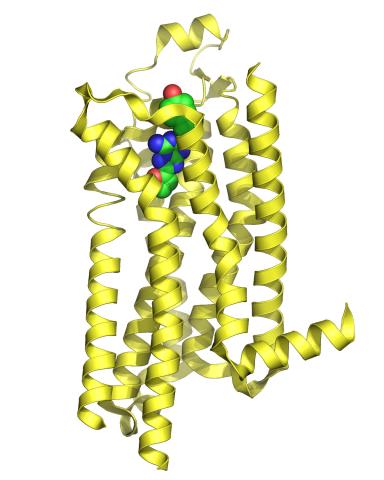
3361: A2A adenosine receptor
3361: A2A adenosine receptor
The receptor is shown bound to an inverse agonist, ZM241385.
Raymond Stevens, The Scripps Research Institute
View Media
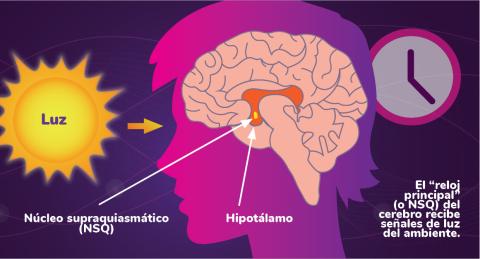
6614: Los ritmos circadianos y el núcleo supraquiasmático
6614: Los ritmos circadianos y el núcleo supraquiasmático
Los ritmos circadianos son cambios físicos, mentales y de comportamiento que siguen un ciclo de 24 horas. Los ritmos circadianos se ven influenciados por la luz y están regulados por el núcleo supraquiasmático del cerebro, a veces denominado el reloj principal.
Vea 6613 para la versión en inglés de esta infografía.
Vea 6613 para la versión en inglés de esta infografía.
NIGMS
View Media
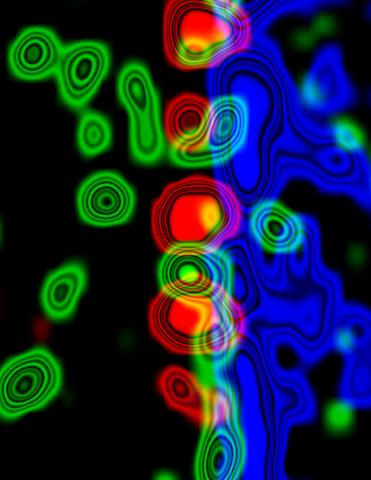
3734: Molecular interactions at the astrocyte nuclear membrane
3734: Molecular interactions at the astrocyte nuclear membrane
These ripples of color represent the outer membrane of the nucleus inside an astrocyte, a star-shaped cell inside the brain. Some proteins (green) act as keys to unlock other proteins (red) that form gates to let small molecules in and out of the nucleus (blue). Visualizing these different cell components at the boundary of the astrocyte nucleus enables researchers to study the molecular and physiological basis of neurological disorders, such as hydrocephalus, a condition in which too much fluid accumulates in the brain, and scar formation in brain tissue leading to abnormal neuronal activity affecting learning and memory. Scientists have now identified a pathway may be common to many of these brain diseases and begun to further examine it to find ways to treat certain brain diseases and injuries.
Katerina Akassoglou, Gladstone Institute for Neurological Disease & UCSF
View Media
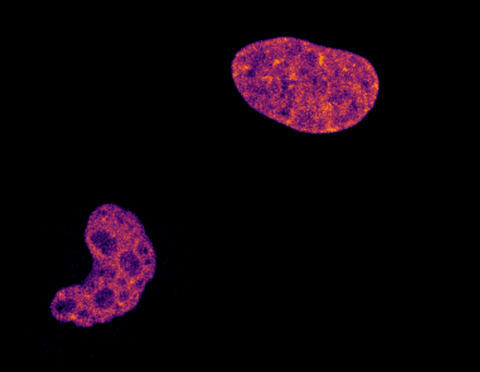
6790: Cell division and cell death
6790: Cell division and cell death
Two cells over a 2-hour period. The one on the bottom left goes through programmed cell death, also known as apoptosis. The one on the top right goes through cell division, also called mitosis. This video was captured using a confocal microscope.
Dylan T. Burnette, Vanderbilt University School of Medicine.
View Media

5810: Tongue 1
5810: Tongue 1
Microscopy image of tongue. One in a series of two, see image 5811
National Center for Microscopy and Imaging Research (NCMIR)
View Media
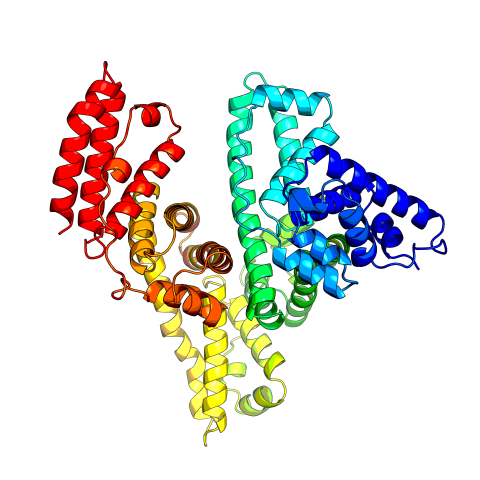
3745: Serum albumin structure 2
3745: Serum albumin structure 2
Serum albumin (SA) is the most abundant protein in the blood plasma of mammals. SA has a characteristic heart-shape structure and is a highly versatile protein. It helps maintain normal water levels in our tissues and carries almost half of all calcium ions in human blood. SA also transports some hormones, nutrients and metals throughout the bloodstream. Despite being very similar to our own SA, those from other animals can cause some mild allergies in people. Therefore, some scientists study SAs from humans and other mammals to learn more about what subtle structural or other differences cause immune responses in the body.
Related to entries 3744 and 3746
Related to entries 3744 and 3746
Wladek Minor, University of Virginia
View Media
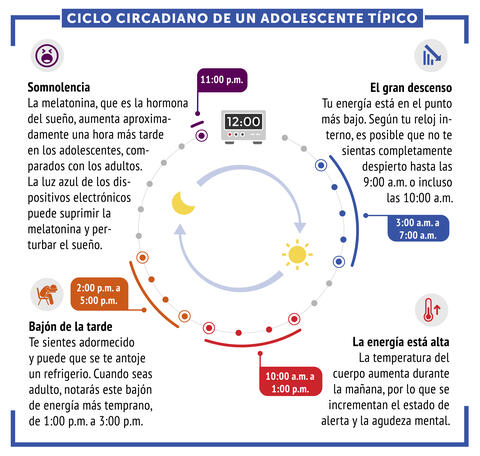
6612: Ciclo circadiano de un adolescente típico
6612: Ciclo circadiano de un adolescente típico
Los ritmos circadianos son cambios físicos, mentales y conductuales que siguen un ciclo de 24 horas. Los ritmos circadianos típicos conducen a un nivel alto de energía durante la mitad del día (de 10 a.m. a 1 p.m.) y un bajón por la tarde. De noche, los ritmos circadianos hacen que la hormona melatonina aumente, lo que hace que la persona se sienta somnolienta.
Vea 6611 para la versión en inglés de esta infografía.
Vea 6611 para la versión en inglés de esta infografía.
NIGMS
View Media
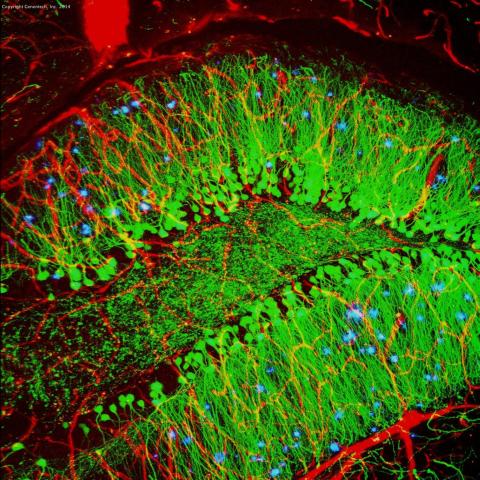
3604: Brain showing hallmarks of Alzheimer's disease
3604: Brain showing hallmarks of Alzheimer's disease
Along with blood vessels (red) and nerve cells (green), this mouse brain shows abnormal protein clumps known as plaques (blue). These plaques multiply in the brains of people with Alzheimer's disease and are associated with the memory impairment characteristic of the disease. Because mice have genomes nearly identical to our own, they are used to study both the genetic and environmental factors that trigger Alzheimer's disease. Experimental treatments are also tested in mice to identify the best potential therapies for human patients.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
Alvin Gogineni, Genentech
View Media
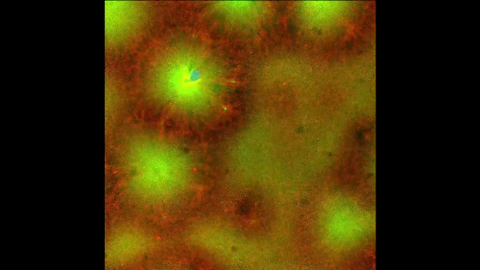
6590: Cell-like compartments emerging from scrambled frog eggs 4
6590: Cell-like compartments emerging from scrambled frog eggs 4
Cell-like compartments that spontaneously emerged from scrambled frog eggs, with nuclei (blue) from frog sperm. Endoplasmic reticulum (red) and microtubules (green) are also visible. Video created using confocal microscopy.
For more photos of cell-like compartments from frog eggs view: 6584, 6585, 6586, 6591, 6592, and 6593.
For videos of cell-like compartments from frog eggs view: 6587, 6588, 6589.
Xianrui Cheng, Stanford University School of Medicine.
View Media
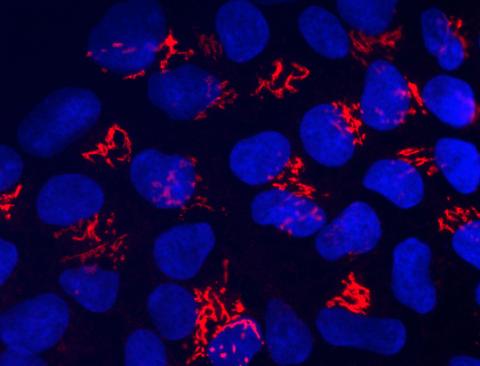
3341: Suicidal Stem Cells
3341: Suicidal Stem Cells
Embryonic stem cells store pre-activated Bax (red) in the Golgi, near the nucleus (blue). Featured in the June 21, 2012, issue of Biomedical Beat.
Mohanish Deshmukh
View Media
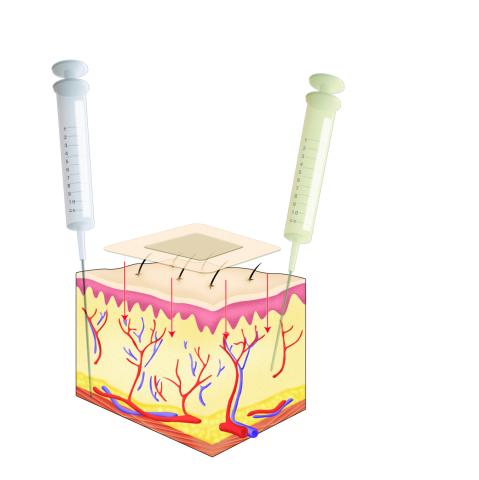
2531: Drugs enter skin
2531: Drugs enter skin
Drugs enter different layers of skin via intramuscular, subcutaneous, or transdermal delivery methods. See image 2532 for a labeled version of this illustration. Featured in Medicines By Design.
Crabtree + Company
View Media
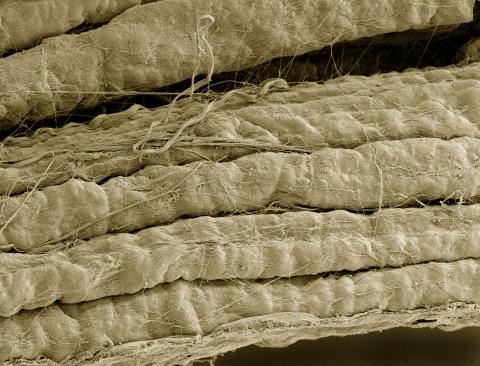
3737: A bundle of myelinated peripheral nerve cells (axons)
3737: A bundle of myelinated peripheral nerve cells (axons)
The extracellular matrix (ECM) is most prevalent in connective tissues but also is present between the stems (axons) of nerve cells. The axons of nerve cells are surrounded by the ECM encasing myelin-supplying Schwann cells, which insulate the axons to help speed the transmission of electric nerve impulses along the axons.
Tom Deerinck, National Center for Microscopy and Imaging Research (NCMIR)
View Media
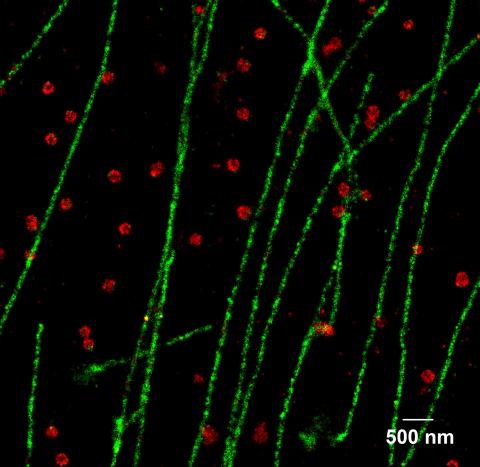
2325: Multicolor STORM
2325: Multicolor STORM
In 2006, scientists developed an optical microscopy technique enabling them to clearly see individual molecules within cells. In 2007, they took the technique, abbreviated STORM, a step further. They identified multicolored probes that let them peer into cells and clearly see multiple cellular components at the same time, such as these microtubules (green) and small hollows called clathrin-coated pits (red). Unlike conventional methods, the multicolor STORM technique produces a crisp and high resolution picture. A sharper view of how cellular components interact will likely help scientists answer some longstanding questions about cell biology.
Xiaowei Zhuang, Harvard University
View Media
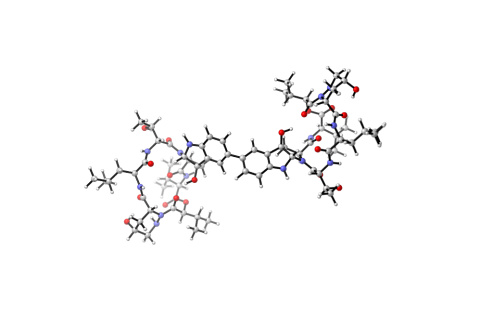
6848: Himastatin
6848: Himastatin
A model of the molecule himastatin, which was first isolated from the bacterium Streptomyces himastatinicus. Himastatin shows antibiotic activity. The researchers who created this image developed a new, more concise way to synthesize himastatin so it can be studied more easily.
More information about the research that produced this image can be found in the Science paper “Total synthesis of himastatin” by D’Angelo et al.
Related to image 6850 and video 6851.
More information about the research that produced this image can be found in the Science paper “Total synthesis of himastatin” by D’Angelo et al.
Related to image 6850 and video 6851.
Mohammad Movassaghi, Massachusetts Institute of Technology.
View Media

1090: Natcher Building 10
1090: Natcher Building 10
NIGMS staff are located in the Natcher Building on the NIH campus.
Alisa Machalek, National Institute of General Medical Sciences
View Media
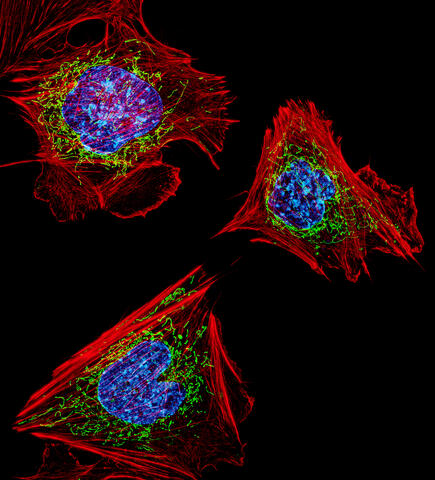
3624: Fibroblasts with nuclei in blue, energy factories in green and the actin cytoskeleton in red
3624: Fibroblasts with nuclei in blue, energy factories in green and the actin cytoskeleton in red
The cells shown here are fibroblasts, one of the most common cells in mammalian connective tissue. These particular cells were taken from a mouse embryo. Scientists used them to test the power of a new microscopy technique that offers vivid views of the inside of a cell. The DNA within the nucleus (blue), mitochondria (green), and actin filaments in the cellular skeleton (red) are clearly visible.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
This image was part of the Life: Magnified exhibit that ran from June 3, 2014, to January 21, 2015, at Dulles International Airport.
Dylan Burnette, NICHD
View Media

2690: Dolly the sheep
2690: Dolly the sheep
Scientists in Scotland were the first to clone an animal, this sheep named Dolly. She later gave birth to Bonnie, the lamb next to her.
View Media
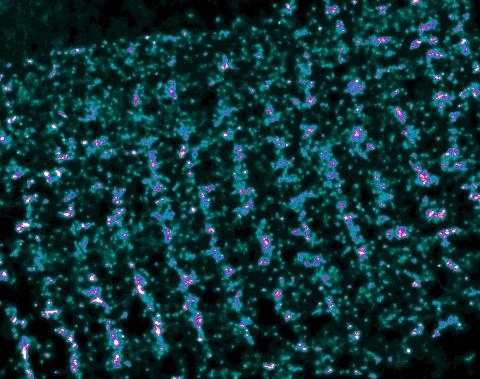
3339: Single-Molecule Imaging
3339: Single-Molecule Imaging
This is a super-resolution light microscope image taken by Hiro Hakozaki and Masa Hoshijima of NCMIR. The image contains highlighted calcium channels in cardiac muscle using a technique called dSTORM. The microscope used in the NCMIR lab was built by Hiro Hakozaki.
Tom Deerinck, NCMIR
View Media
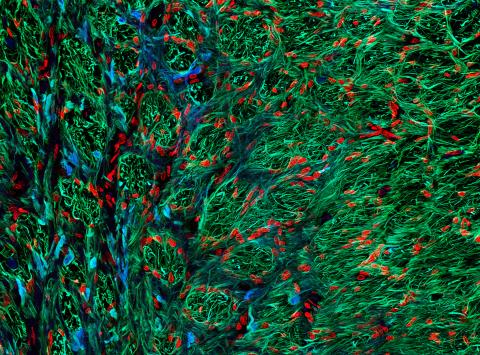
5852: Optic nerve astrocytes
5852: Optic nerve astrocytes
Astrocytes in the cross section of a human optic nerve head
Tom Deerinck and Keunyoung (“Christine”) Kim, NCMIR
View Media
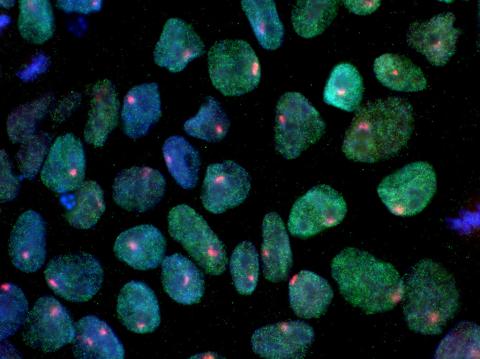
3279: Induced pluripotent stem cells from skin 02
3279: Induced pluripotent stem cells from skin 02
These induced pluripotent stem cells (iPS cells) were derived from a woman's skin. Blue show nuclei. Green show a protein found in iPS cells but not in skin cells (NANOG). The red dots show the inactivated X chromosome in each cell. These cells can develop into a variety of cell types. Image and caption information courtesy of the California Institute for Regenerative Medicine. Related to image 3278.
Kathrin Plath lab, University of California, Los Angeles, via CIRM
View Media
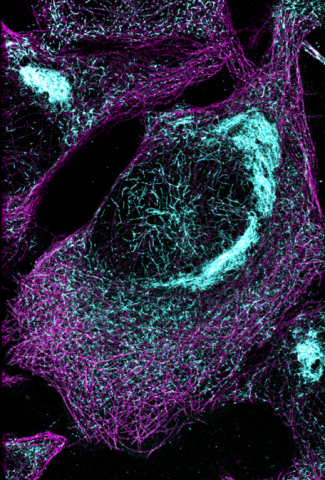
6892: Microtubules and tau aggregates
6892: Microtubules and tau aggregates
Microtubules (magenta) and tau protein (light blue) in a cell model of tauopathy. Researchers believe that tauopathy—the aggregation of tau protein—plays a role in Alzheimer’s disease and other neurodegenerative diseases. This image was captured using Stochastic Optical Reconstruction Microscopy (STORM).
Related to images 6889, 6890, and 6891.
Related to images 6889, 6890, and 6891.
Melike Lakadamyali, Perelman School of Medicine at the University of Pennsylvania.
View Media

5753: Clathrin-mediated endocytosis
5753: Clathrin-mediated endocytosis
Endocytosis is the process by which cells are able to take up membrane and extracellular materials through the formation of a small intracellular bubble, called a vesicle. This process, called membrane budding, is generally by a coating of proteins. This protein coat helps both to deform the membrane and to concentrate specific proteins inside the newly forming vesicle. Clathrin is a coat protein that functions in receptor-mediated endocytosis events at the plasma membrane. This animation shows the process of clathrin-mediated endocytosis. An iron-transport protein called transferrin (blue) is bound to its receptor (purple) on the exterior cell membrane. Inside the cell, a clathrin cage (shown in white/beige) assembles through interactions with membrane-bound adaptor proteins (green), causing the cell membrane to begin bending. The adaptor proteins also bind to receptors for transferrin, capturing them in the growing vesicle. Molecules of a protein called dynamin (purple) are then recruited to the neck of the vesicle and are involved in separating the membranes of the cell and the vesicle. Soon after the vesicle has budded off the membrane, the clathrin cage is disassembled. This disassembly is mediated by another protein called HSC70 (yellow), and its cofactor protein auxilin (orange).
Janet Iwasa, University of Utah
View Media
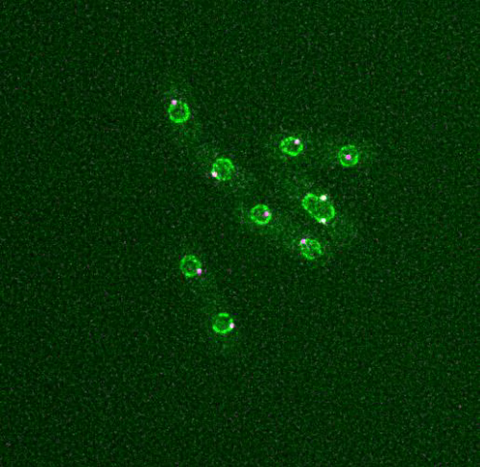
6795: Dividing yeast cells with nuclear envelopes and spindle pole bodies
6795: Dividing yeast cells with nuclear envelopes and spindle pole bodies
Time-lapse video of yeast cells undergoing cell division. Nuclear envelopes are shown in green, and spindle pole bodies, which help pull apart copied genetic information, are shown in magenta. This video was captured using wide-field microscopy with deconvolution.
Related to images 6791, 6792, 6793, 6794, 6797, 6798, and video 6796.
Related to images 6791, 6792, 6793, 6794, 6797, 6798, and video 6796.
Alaina Willet, Kathy Gould’s lab, Vanderbilt University.
View Media
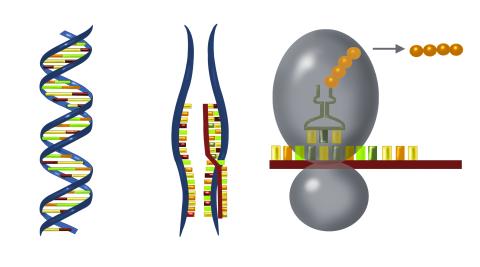
2547: Central dogma, illustrated
2547: Central dogma, illustrated
DNA encodes RNA, which encodes protein. DNA is transcribed to make messenger RNA (mRNA). The mRNA sequence (dark red strand) is complementary to the DNA sequence (blue strand). On ribosomes, transfer RNA (tRNA) reads three nucleotides at a time in mRNA to bring together the amino acids that link up to make a protein. See image 2548 for a labeled version of this illustration and 2549 for a labeled and numbered version. Featured in The New Genetics.
Crabtree + Company
View Media
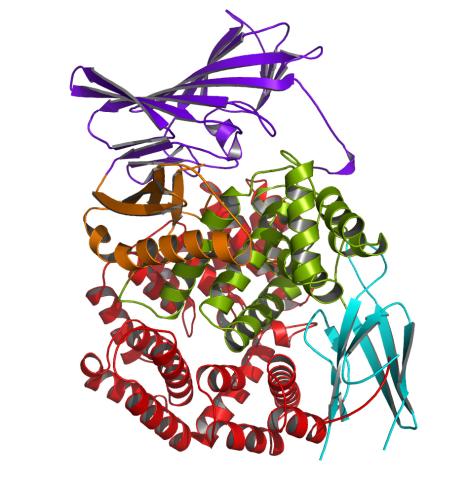
2341: Aminopeptidase N from N. meningitidis
2341: Aminopeptidase N from N. meningitidis
Model of the enzyme aminopeptidase N from the human pathogen Neisseria meningitidis, which can cause meningitis epidemics. The structure provides insight on the active site of this important molecule.
Midwest Center for Structural Genomics, PSI
View Media
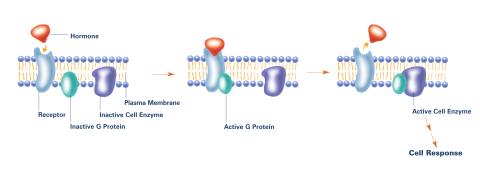
2537: G switch (with labels)
2537: G switch (with labels)
The G switch allows our bodies to respond rapidly to hormones. G proteins act like relay batons to pass messages from circulating hormones into cells. A hormone (red) encounters a receptor (blue) in the membrane of a cell. Next, a G protein (green) becomes activated and makes contact with the receptor to which the hormone is attached. Finally, the G protein passes the hormone's message to the cell by switching on a cell enzyme (purple) that triggers a response. See image 2536 and 2538 for other versions of this image. Featured in Medicines By Design.
Crabtree + Company
View Media
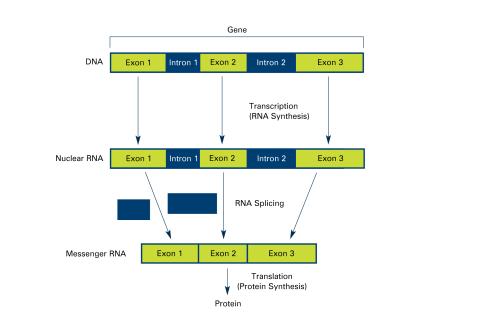
2551: Introns (with labels)
2551: Introns (with labels)
Genes are often interrupted by stretches of DNA (introns, blue) that do not contain instructions for making a protein. The DNA segments that do contain protein-making instructions are known as exons (green). See image 2550 for an unlabeled version of this illustration. Featured in The New Genetics.
Crabtree + Company
View Media